Hydrogels for the study of cancer and fibrosis
In this piece, Dr Matthew Walker summarises the importance of two recently published reviews on the topic of mechanobiology in both cancer and fibrosis, both of which can be accessed here:
- Walker M, Morton JP. Hydrogel models of pancreatic adenocarcinoma to study cell mechanosensing. Biophysical Reviews. 2024;16(6):851-70. (doi: 10.1007/s12551-024-01265-8)
- Walker M, Gourdon D, Cantini M. Beyond static models: Mechanically dynamic matrices reveal new insights into cancer and fibrosis progression. Current Opinion in Biomedical Engineering. 2025;33:100570. (doi: 10.1016/j.cobme.2024.100570)
In cancer and fibrosis, the mechanical properties of tissues change dramatically largely due to diseased cells producing abnormal amounts of extracellular matrix (ECM). The ECM is crucial for regulating the behaviour of cells as they interact with it; these significant changes are speculated to play a key role in progressing cancer and fibrosis. In my research, I am very interested in understanding how these changes in mechanical properties regulate the behaviour of cells in disease through various biochemical mechanisms that can be grouped under a term known as mechanobiology. This is important to understand as it will help scientists and clinicians to identify new diagnostic and therapeutic targets that could lead to improved patient outcomes.
In these recent reviews, we describe how recent studies (within the last 5 years) have highlighted the importance of changes in the mechanical properties that occur during cancer and fibrosis and how these changes cause abnormal cell behaviour which leads to disease progression. We focus on how researchers have developed lab models of cancer and fibrosis tissue to study these effects in a controlled environment. This has been achieved using hydrogels which are jelly-like materials that are very similar to the ECM properties of cancer and fibrosis tissue. Importantly, the mechanical properties of hydrogels can be easily controlled to mimic that of disease tissue which is critical to understand how cells respond to these properties in disease.
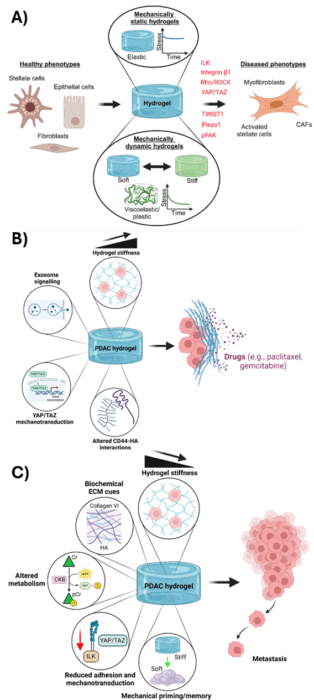
Figure 1: Using hydrogels in cancer and fibrosis to understand how mechanobiology is important in how diseases progress.
Recent studies have shown that if the mechanical properties of hydrogels are similar to cancer and fibrosis tissue, this activates the mechanobiology of cells and causes many processes to occur which are associated with disease progression, highlighted in Figure 1. I found that 3 key events were heavily linked to altered cell mechanobiology: differentiation, chemoresistance and metastasis.
A) Differentiation involves cells transforming from one type of cell to another which is a normal process in human biology; in cancer and fibrosis however, this process is hijacked and causes cells to behave abnormally which promotes disease progression.
(B) Chemoresistance involves the increased resistance of tumours to chemotherapeutic drugs; this is often due to a dense tumour ECM forming around the tissue making drugs more impermeable.
(C) Metastasis involves the migration of cancer cells to other sites in the body after the initial primary tumour has formed; this is associated with a much poorer prognosis for patients.
Collectively, these reviews highlight the importance of developing hydrogels to study how mechanobiology is critical to regulate many aspects of disease progression in cancer and fibrosis.