Integrin-growth factor dynamics in cancer stemness and tissue regeneration
Dr Udesh Dhawan introduces some of his recent research on how integrins can be utilised in growth factor activation and the development of a mechanobiology-based bone regeneration platform.
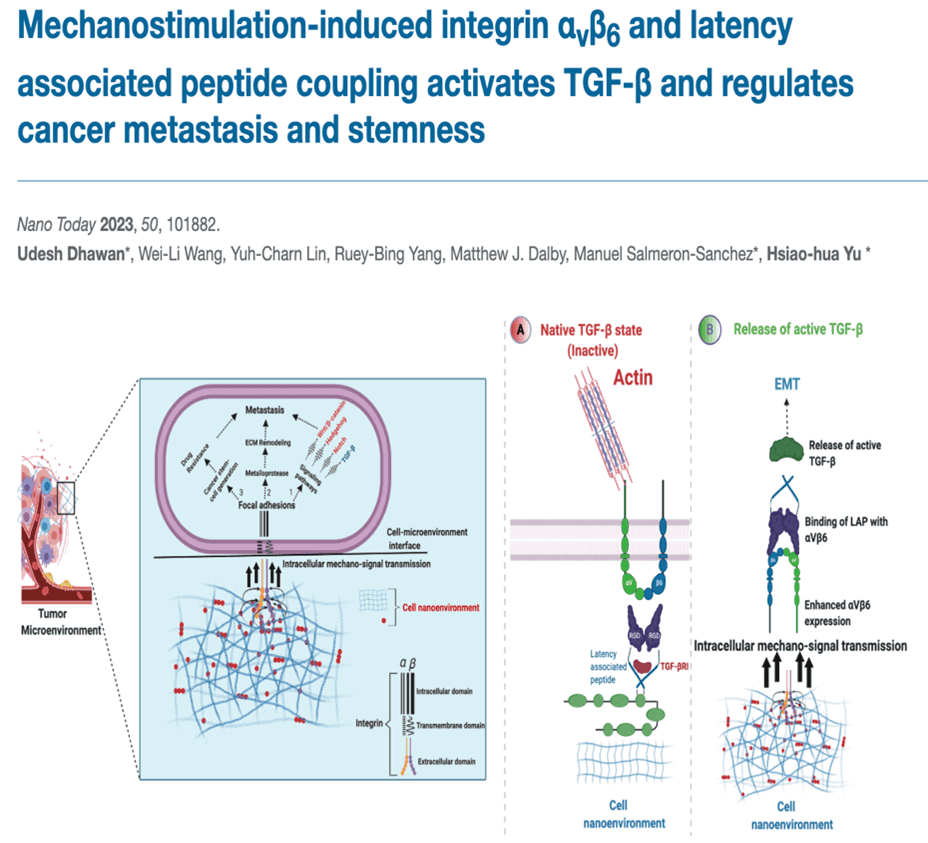
Integrins are specialised adhesion proteins that enable cells to attach and survive. Take away integrins, and we take away the ability of cells that rely on adhesion for survival. Without integrins, we are left with floating cell bodies that can not be used for any in-vitro experimental validations. To achieve cell attachment, integrins bind with several extracellular matrix (ECM) proteins. In doing so, they also drive activation of growth factors like Transforming growth factor Beta (TGFβI) which control stem cell differentiation into bone-forming cells. The involvement of integrins in cell attachment, proliferation and differentiation also makes them an integral part of normal and abnormal processes such as tissue regeneration and cancer origination, respectively.
My work over the years has shown that integrins play crucial role in activating TGFβI and imparting stemness abilities to bone cancer cells (Dhawan U., Salmeron-Sanchez M. et al., Nano Today, 50, 101882, 2023). On the bright side, I have also capitalised on the ability of integrins to activate growth factors to engineer a bone regeneration platform that allows mesenchymal stem cells to decide how much of TGFβI do they want to activate for regenerating bone defects (Dhawan U., Salmeron-Sanchez, M., et al., Advanced Materials, 36, 2310789, 2024). To enable this, I developed a novel strategy using a small latent TGFβI-binding peptide (LTBPI) to trap the inactive form of TGFβI (LAP) in the ECM. By adjusting LTBPI concentration, I controlled the amount of LAP available for integrin (βI)-mediated traction. This traction pulled on RGD domains flanking LAP, releasing active TGFβI. In essence, I regulated cell contractility to modulate TGFβI activation.
This world’s first mechanobiology-based bone regeneration platform presents an interesting epiphany – to be able to utilise integrins for desired application, we need to pay close attention to the surface properties of the implants. To validate surface-dependent interactions of integrins with ECM proteins and growth factors, I utilise immunoblotting and co-immunoprecipitation techniques which are considered the gold-standard when investigating protein expression and protein-protein interactions. My current work heavily relies on these techniques to answer a basic but important question – why do cells stay round with impaired cell division on some surfaces but spread and proliferate well on others? I anticipate that answering this question will underpin material strategies for future biomaterials.