Harnessing the mechanosensitivity of cells for regenerative therapies and disease detection
Dr Olivia Johnson-Love introduces the novel method of vibration-based cell engineering in order to alter the fate of stem cells.
Cells are highly responsive to mechanical cues in their microenvironment, modulating their behaviour through proliferation, differentiation, or morphological adaptation. This mechanosensitivity has been widely studied for directing stem cell differentiation and offers promising avenues for disease diagnosis, as pathological cells may exhibit altered responses to mechanical stimuli compared to healthy cells.
My research focuses on understanding how vibrational stimulation influences cellular behaviour, with a particular interest in its applications in regenerative medicine and diagnostics. While vibrational stimulation has been shown to promote the differentiation of stem cells into osteogenic, chondrogenic, and adipogenic lineages, there is considerable inconsistency in the literature regarding optimal vibration parameters. Our recent review (Johnson-Love, O., Salmeron-Sanchez, M., Reid, S. et al. Nat Rev Bioeng, 2025) highlights the variability in experimental approaches and the lack of consensus on effective vibration conditions.
To address this, I conducted a study evaluating how frequency, amplitude, direction, and duration of vibration influence cellular responses. Building on previous protocols that applied 30 nm amplitude vibration at 1 kHz over 28 days, I investigated how cells respond to variations in these mechanical conditions (Figure 1). I found that higher amplitude, horizontally applied vibration enhanced osteogenic differentiation, increasing the expression of osteogenic markers, alongside increases in nuclear area and actin intensity.
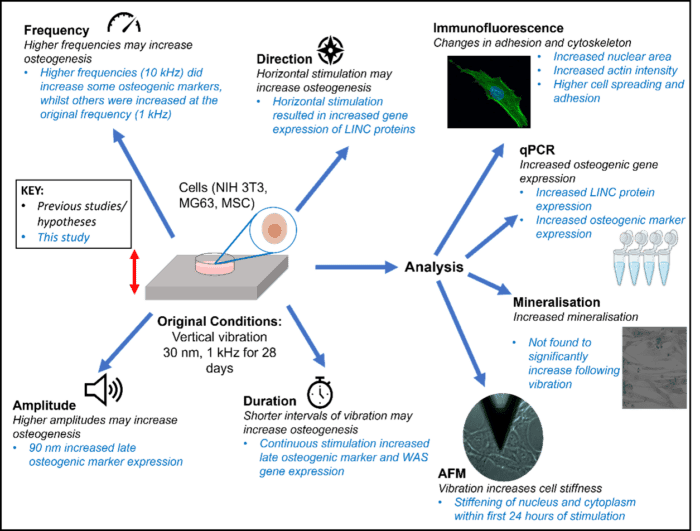
Figure 1: Summary of key findings from the study, highlighting the four main parameters explored and the primary analyses conducted to assess cellular responses to vibration.
Notably, using the original conditions of 30 nm amplitude at 1 kHz, I observed increased nuclear and cytoplasmic stiffness within 24 hours of stimulation. This rapid mechanical adaptation further highlights the sensitivity of cells to vibrational input and supports the potential of leveraging these responses for diagnostic applications. These findings underscore the potential of using cellular mechanical response – particularly to vibration – as a diagnostic tool. My current work explores whether disease specific alterations in mechanosensitivity can be leveraged to distinguish healthy from pathological cells, offering a novel route for early detection and therapeutic monitoring.