-
The bone marrow is a site of health and disease. In health, it produces all of the blood cells that we rely on to carry oxygen and protect us from infection. However, the stem cells that produce the blood and that reside in the marrow, the haematopoietic stem cells (HSCs), age and can tip over into disease states, such as developing leukaemia. Factors such as smoking and treatment of cancers elsewhere in the body (toxic effects of chemotherapy/radiotherapy) can accelerate ageing, and therefore, drive the transition to disease. Further, it forms a home to other cancer cells, that leave their original tumour and move, or metastasise, to the bone marrow. Once in the marrow, they can become dormant, hiding from chemotherapies and activating sometime later to form devastating bone cancers. The cues that wake cancer cells from dormancy are largely unknown.
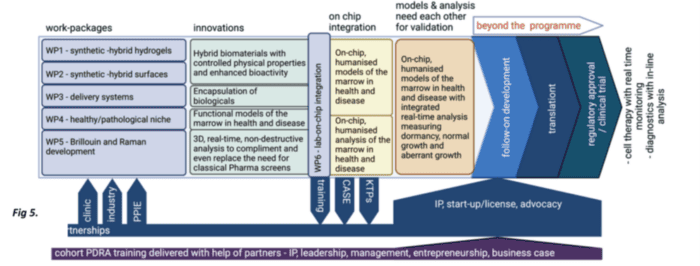
If models of the bone marrow that contain human cells and that can mimic key facets of the niche in the lab, such as blood regeneration, cancer evolution and dormancy, can be developed it would be a big help in the search for better cancer therapies. We are developing the materials and technologies required to meet this challenge. In this programme of research, we will tackle three biomedical challenges:
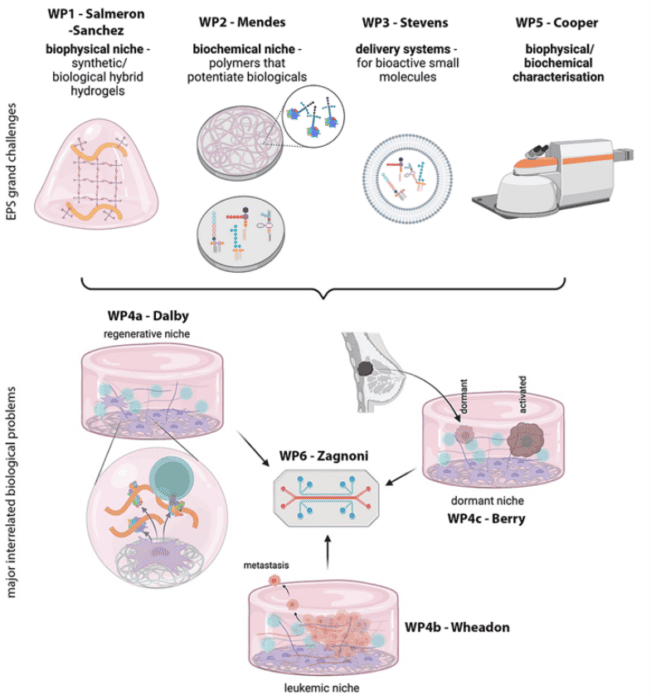
1) HSC regeneration. Bone marrow transplantation (more correctly HSC transplantation) is a one-donor, one-recipient therapy that can be curative for blood diseases such as leukaemia. It is limited as HSCs cannot be looked after well out of the body. Approaches to properly look after these precious cells in the lab could allow this key therapy to become a onedonor, multiple recipient treatment. Further, the ability to look after the cells in the lab would open up the potential for genetically modifying the cells to allow us to cure the cells and put them back into the patient, losing the need for patient immunosuppression.
2) Cancer evolution. As we get older, our cells collect mutations in their DNA and these mutations can be drivers of cancer. Lifestyle choices such as smoking, and side effects of treatments of other diseases can also add mutations to the cells. As blood cancers develop, the bone marrow changes its architecture to protect these diseased HSCs. Our 3D environments will allow us to better understand this marrow remodelling process and how drugs can target cancers in this more protective environment. The models will also allow us to study the potential toxicity of gene-edited HSCs to make sure they don’t produce unwanted side effects or are not cancerous in themselves.
3) Dormancy. What triggers dormancy and activation from dormancy are poorly understood. By placing our 3D environments in a miniaturised format where we can connect other models that include infection and immune response, we can start to understand the factors involved in the activation of cancer cells from dormancy.
Our vision is driven by materials and engineering, as the bone marrow niche is rich in structural and signalling biological materials (proteins). Therefore, we will establish three engineering challenges:
(1) Cells can be controlled by the stiffness and viscous nature of materials (viscoelasticity). We will therefore develop synthetic-biological hybrid materials that can be manufactured to have reproducible physical properties and that have biological functionality.
(2) We will develop these materials to interact with growth factors and bioactive metabolites, both of which are powerful controllers of cell behaviours. These materials will be used to assemble the HSC microenvironments in lab-on-chip (miniaturised) format to allow high-content drug and toxicity screening.
(3) We will develop real-time systems to detect changes in cell behaviour, such as the transition from health to cancer using Raman and Brillouin microscopies.
The use of animals in research provides poor predictivity. We will offer better than animal model alternatives.